CBSE Class 12 Chemistry Practical Syllabus 2022-23: CBSE Class 12 Chemistry Practicals contribute 30 marks towards the annual assessment. Students can easily score good marks in practicals with regular practice and an aware mind. Students must learn the identification of different chemicals and to perform the experiments prescribed in the CBSE practical syllabus.
We have provided here the CBSE Class 12 Chemistry Practical Syllabus 2022-2023 for students to download in PDF. With this latest syllabus, students would get to know the practical evaluation scheme and the list of experiments for the current academic session. They must check the guidelines and instructions mentioned in the syllabus and prepare for their practical exam accordingly.
CBSE Class 12 Chemistry Practical Syllabus 2022-23
Evaluation Scheme
Check CBSE Class 12 Chemistry Practical Evaluation Scheme 2022-2023 below:
3 HOURS/ 30 MARKS
| Evaluation Scheme for Examination | Marks |
| Volumetric Analysis | 08 |
| Salt Analysis | 08 |
| Content Based Experiment | 06 |
| Project Work | 04 |
| Class record and viva | 04 |
| Total | 30 |
CBSE Class 12 Chemistry Practical Syllabus Content 2022-23
Micro-chemical methods are available for several of practical experiments. Wherever possible, such techniques should be used.
A.Surface Chemistry
(a) Preparation of one lyophilic and one lyophobic sol Lyophilic sol - starch, egg albumin and gum Lyophobic sol - aluminium hydroxide, ferric hydroxide, arsenous sulphide.
(b) Dialysis of sol-prepared in (a) above.
(c) Study of the role of emulsifying agents in stabilizing the emulsion of different oils.
B.Chemical Kinetics
(a) Effect of concentration and temperature on the rate of reaction between Sodium Thiosulphate and Hydrochloric acid.
(b) Study of reaction rates of any one of the following:
(i) Reaction of Iodide ion with Hydrogen Peroxide at room temperature using different concentrations of Iodide ions.
(ii) Reaction between Potassium Iodate, (KIO3) and Sodium Sulphite: (Na2SO3) using starch solution as an indicator (clock reaction).
C.Thermochemistry
Any one of the following experiments
- Enthalpy of dissolution of Copper Sulphate or Potassium Nitrate.
- (b) Enthalpy of neutralization of strong acid (HCI) and strong base (NaOH).
- (c) Determination of enthaply change during interaction (Hydrogen bond formation) between Acetone and Chloroform.
D. Electrochemistry
- Variation of cell potential in Zn/Zn2+|| Cu2+/Cu with change in concentration of electrolytes (CuSO4 or ZnSO4) at room temperature.
E.Chromatography
(a) Separation of pigments from extracts of leaves and flowers by paper chromatography and determination of Rf values.
(b) Separation of constituents present in an inorganic mixture containing two cations only (constituents having large difference in Rf values to be provided).
F.Preparation of Inorganic Compounds
Preparation of double salt of Ferrous Ammonium Sulphate or Potash Alum. Preparation of Potassium Ferric Oxalate.
G.Preparation of Organic Compounds
Preparation of any one of the following compounds
i) Acetanilide ii) Di -benzalAcetone iii) p-Nitroacetanilide iv) Aniline yellow or 2 - Naphthol Anilinedye.
H.Tests for the functional groups present in organic compounds:
Unsaturation, alcoholic, phenolic, aldehydic, ketonic, carboxylic and amino (Primary) groups.
I.Characteristic tests of carbohydrates, fats and proteins in pure samples and their detection in given foodstuffs.
J.Determination of concentration/ molarity of KMnO4 solution by titrating it against a standard solution of:
(a) Oxalic acid,
(b) Ferrous Ammonium Sulphate (Students will be required to prepare standard solutions by weighing themselves).
K.Qualitative analysis
Determination of one anion and one cation in a given salt
Cation:
Pb2+, Cu2+ As3+, Al3+ , Fe3+, Mn2+, Zn2+, Ni2+, Ca2+, Sr2+, Ba2+, Mg2+, NH4+
Anions:
(CO3) 2- , S2- , (SO3) 2- , (NO2) - , (SO4) 2- , Cℓ- , Br- , I- , (PO4) 3- , (C2O4) 2- , CH3COO- , NO3 - (Note: Insoluble salts excluded)
INVESTIGATORY PROJECT
Scientific investigations involving laboratory testing and collecting information from other sources.
A few suggested Projects:
- Study of the presence of oxalate ions in guava fruit at different stages of ripening.
- Study the quantity of casein present in different samples of milk.
- Preparation of soybean milk and its comparison with natural milk with respect to curd formation, the effect of temperature, etc.
- Study of the effect of Potassium Bisulphate as a food preservative under various conditions (temperature, concentration, time, etc.)
- Study of digestion of starch by salivary amylase and effect of pH and temperature on it.
- Comparative study of the rate of fermentation of the following materials: wheat flour, gram flour, potato juice, carrot juice, etc.
- Extraction of essential oils present in Saunf (aniseed), Ajwain (carum), Illaichi (cardamom).
- Study of common food adulterants in fat, oil, butter, sugar, turmeric power, chilli powder and pepper.
Note: Any other investigatory project, which involves about 10 periods of work, can be chosen with the approval of the teacher. Practical Examination for Visually Impaired Students of Classes XI and XII Evaluation Sch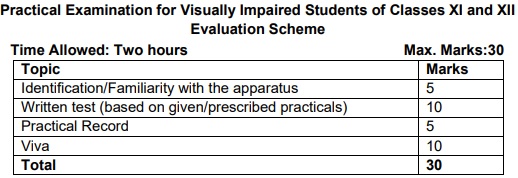
CBSE Class 12 Chemistry Practical 2022-23: General Guidelines
- The practical examination will be of two hours duration.
- A separate list of ten experiments is included here.
- The written examination in practicals for these students will be conducted at the time of the practical examination of all other students.
- The written test will be of 30 minutes duration.
- The question paper given to the students should be legibly typed. It should contain a total of 15 practical skill-based very short answer type questions. A student would be required to answer any 10 questions.
- A writer may be allowed to such students as per CBSE examination rules.
- All questions included in the question papers should be related to the listed practical. Every question should require about two minutes to be answered.
- These students are also required to maintain a practical file. A student is expected to record at least five of the listed experiments as per the specific instructions for each subject. These practicals should be duly checked and signed by the internal examiner.
- The format of writing any experiment in the practical file should include aim, apparatus required, simple theory, procedure, related practical skills, precautions etc.
- Questions may be generated jointly by the external/internal examiners and used for assessment.
- The viva questions may include questions based on basic theory/principle/concept, apparatus/materials/ chemicals required, procedure, precautions, sources of error etc.
1.Items for Identification/Familiarity of the apparatus for assessment in practical (All experiments)
Beaker, glass rod, tripod stand, wire gauze, Bunsen burner, Whatman filter paper, gas jar, capillary tube, pestle and mortar, test tubes, tongs, test tube holder, test tube stand, burette, pipette, conical flask, standard flask, clamp stand, funnel, filter paper
Hands-on Assessment
- Identification/familiarity with the apparatus
- Odour detection in qualitative analysis
2.List of Practicals
The experiments have been divided into two sections:
Section A and Section B.
The experiments mentioned in Section B are mandatory.
SECTION- A
A Surface Chemistry
1 Preparation of one lyophilic and one lyophobic sol - starch, egg albumin and gum
2 Preparation of one lyophobic sol– Ferric hydroxide
B Chromatography
Separation of pigments from extracts of leaves and flowers by paper chromatography and determination of Rf values (distance values may be provided).
C Tests for the functional groups present in organic compounds:
(1) Alcoholic and Carboxylic groups.
(2) Aldehydic and Ketonic
D Characteristic tests of carbohydrates and proteins in the given foodstuffs.
E Preparation of Inorganic Compounds- Potash Alum
SECTION-B (Mandatory)
F Quantitative analysis
(1) (a) Preparation of the standard solution of Oxalic acid of a given volume
(b) Determination of molarity of KMnO4 solution by titrating it against a standard solution of Oxalic acid.
(2) The above exercise [F 1 (a) and (b)] to be conducted using Ferrous ammonium sulphate (Mohr's salt)
G Qualitative analysis:
(1) Determination of one cation and one anion in a given salt.
Cation –NH4+
Anions – CO32- , S2- , SO32- , Cl- , CH3COO-
(Note: Insoluble salts excluded)
Note: The above practical may be carried out in an experiential manner rather than recording observations.
Download the above syllabus in PDF from the following link:
All the best!
Related Syllabus:
Related Sample Paper:
