ICSE Chemistry Specimen Paper 2024 for Class 10: CISCE (Council for the Indian School Certificate Examinations) is a well-known national-level board of education in India. It administers ICSE (Indian Certificate of Secondary Education) for Class 10 and ISC (Indian School Certificate) for Class 12. CISCE was founded in 1958. It is known for its extensive curriculum that emphasises holistic development and equips students for difficulties in higher education. CISCE's educational programmes emphasise critical thinking, problem-solving, and practical skills. The board ensures high academic standards and encourages a well-rounded education through its emphasis on co-curricular activities and community service. CISCE-certified students are recognised globally for their proficiency and competence.
Read: ICSE, ISC Revised Syllabus PDFs 2023-24
This article is about to provide you with insights on the latest ICSE Class 10 Chemistry sample papers for the 2024 ICSE Board exams. This specimen cum sample paper was released by CISCE on its official website after an announcement on July 5, 2023. As per CISCE, you will not find any change in the rubrics or paper pattern. Thus, refer to the previous year's papers and the Chemistry sample paper for Class 10 ICSE.
ICSE Class 10 Chemistry Sample Paper General Guidelines
As per the update from CISCE, the ICSE Class 10 Chemistry paper will follow the below-mentioned guidelines.
- Maximum Marks: 80
- Time allowed: Two hours
- Answers to this Paper must be written on the paper provided separately.
- You will not be allowed to write during first 15 minutes.
- This time is to be spent in reading the question paper.
- The time given at the head of this Paper is the time allowed for writing the answers.
- Section A is compulsory. Attempt any four questions from Section B.
- The intended marks for questions or parts of questions are given in brackets [ ].
ICSE Class 10 Chemistry Specimen Paper 2024
SECTION A (Attempt all questions from this Section.)
Question 1
Choose the correct answers to the questions from the given options. (Do not copy the question, write the correct answers only.)
(i) An aqueous solution of copper sulphate turns colourless on electrolysis. Which of the following could be the electrodes? P. anode: copper; cathode: copper Q. anode: platinum; cathode: copper R. anode: copper; cathode: platinum
(a) only P
(b) only Q
(c) only R
(d) both Q and R
(ii) A compound P is heated in a test tube with sodium hydroxide solution. A red litmus paper held at the mouth of the test tube turns blue. Which of the following could compound P be?
(a) zinc sulphate
(b) copper sulphate
(c) ferrous sulphate
(d) ammonium sulphate
(iii) The atomic masses of sulphur (S), oxygen (O), and helium (He) are approximately 32, 16, and 4 respectively. Which of the following statements regarding the number of atoms in 32 g of sulphur, 16 g of oxygen, and 4 g of helium is correct? P. 16 g of oxygen contains four times the number of atoms as 4 g of helium. Q. 16 g of oxygen contains half the number of atoms as 32 g of sulphur.
(a) only P
(b) only Q
(c) both P and Q
(d) neither P nor Q
(iv) Ammonia gas is passed through quicklime and then collected in a jar. Red and blue litmus papers are placed in the jar. W, X, Y and Z are the four observations. Which of the above observations correctly shows the reaction of the litmus papers to ammonia?
| Red litmus paper | Blue litmus paper | |
| W | turns blue | remains blue |
| X | remains red | remains blue |
| Y | remains red | turns red |
| Z | turns blue | turns red |
(a) W
(b) X
(c) Y
(d) Z
(v) Glucose reacts with concentrated sulphuric acid to give a very pure form of carbon called sugar charcoal. The reaction taking place is:
(a) oxidation
(b) combustion
(c) dehydration
(d) combination
(vi) In which of the following electrolytic cells [P, Q, R or S] will silver plating be done on the spoon?
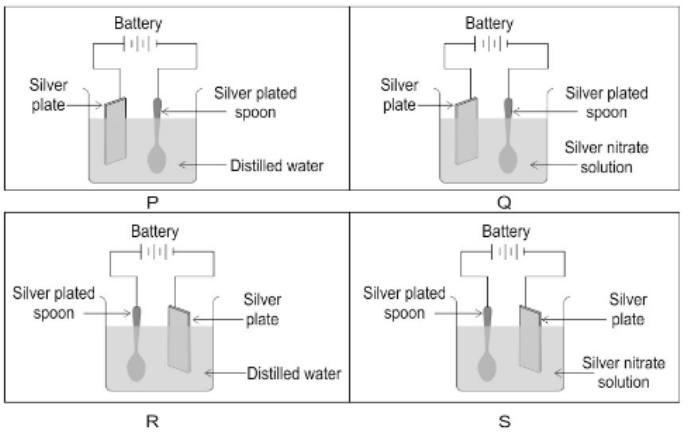
(a) P
(b) Q
(c) R
(d) S
(vii) The basicity of acetic acid is:
(a) 1
(b) 2
(c) 3
(d) 4
(viii) A→A+3; B→B-1 Number of electrons present in the outermost shell of atoms A and B respectively are:
(a) 5, 1
(b) 3, 1
(c) 3, 7
(d) 5, 7
(ix) A __________ solution is observed after placing Magnesium metal in a solution of Copper sulphate for half an hour.
(a) Blue
(b) Colourless
(c) Reddish brown
(d) Dirty green
(x) An element with atomic no. __________ will form an acidic oxide.
(a) 3
(b) 17
(c) 11
(d) 13
(xi) Which of the following is NOT true with respect to nitric acid?
(a) It is a strong reducing agent
(b) It is a strong oxidizing agent
(c) It is unstable to heat
(d) It liberates sulphur dioxide gas when treated with potassium sulphite
(xii) __________ is the functional group in methanol.
(a) >C=O
(b) –OH
(c) –CHO
(d) –COOH
(xiii) The process of electrolysis is an example of:
(a) Oxidation reaction
(b) Reduction reaction
(c) Redox reaction
(d) Displacement reaction
Read: ICSE, ISC All Subject Sample Papers 2024
(xiv) The catalyst used in Ostwald’s process is ___________.
(a) Finely divided iron
(b) Graphite
(c) Vanadium pentoxide
(d) Platinum
(xv) An element belongs to third period and sixteenth group. It will have __________ electrons in its valence shell.
(a) 2
(b) 5
(c) 6
(d) 3
